
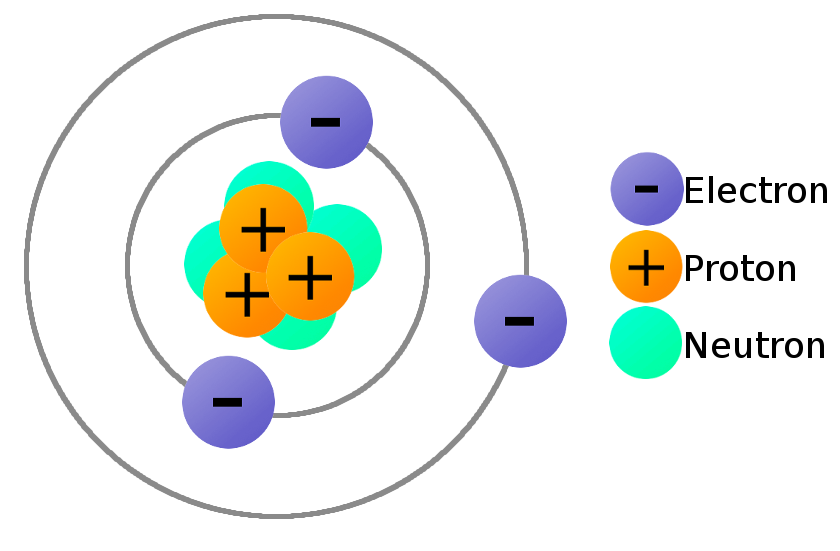
If an atom has 12 protons (atomic number = 12), it must be magnesium. So if an atom has 8 protons (atomic number = 8), it must be oxygen. The atomic number is tied to the position of the element in the Periodic Table and therefore the number of protons defines what sort of element you are talking about. If there are 9 protons, there must be 10 neutrons for the total to add up to 19. The atomic number counts the number of protons (9) the mass number counts protons + neutrons (19). How many protons and neutrons has this atom got? This information can be given simply in the form: The mass number is also called the nucleon number. No of protons + no of neutrons = MASS NUMBER of the atom The atomic number is also given the more descriptive name of proton number. No of protons = ATOMIC NUMBER of the atom Working out the numbers of protons and neutrons Virtually all the mass of the atom is concentrated in the nucleus, because the electrons weigh so little. Protons and neutrons are collectively known as nucleons. The nucleus is at the centre of the atom and contains the protons and neutrons. This actually produces more useful information about both masses and charges than the constant energy version. If in doubt, I suggest you use the second (constant speed) version.
#Simple diagrams of atoms how to#
Information about how to do this is on the syllabuses page. You should look in detail at past questions, mark schemes and examiner's reports which you can get from your examiners if you are doing a UK-based syllabus. If this is on your syllabus, it is important that you should know which version your examiners are going to expect, and they probably won't tell you in the syllabus. Note: This is potentially very confusing! Most chemistry sources that talk about this give either one or the other of these two diagrams without any comment at all - they don't specifically say that they are using constant energy or constant speed beams. If the electrons and protons are travelling with the same speed, then the lighter electrons are deflected far more strongly than the heavier protons. If beams of the three sorts of particles, all with the same speed, are passed between two electrically charged plates: If the electric field was strong enough, then the electron and proton beams might curve enough to hit their respective plates. The amount of deflection is exactly the same in the electron beam as the proton beam if the energies are the same - but, of course, it is in the opposite direction. Protons are deflected on a curved path towards the negative plate.Įlectrons are deflected on a curved path towards the positive plate. If beams of the three sorts of particles, all with the same energy, are passed between two electrically charged plates:
Neutrons don't have a charge, and so would continue on in a straight line.Įxactly what happens depends on whether the beams of particles enter the electric field with the various particles having the same speeds or the same energies Protons are positively charged and so would be deflected on a curving path towards the negative plate.Įlectrons are negatively charged and so would be deflected on a curving path towards the positive plate. What happens if a beam of each of these particles is passed between two electrically charged plates - one positive and one negative? Opposites will attract. The behaviour of protons, neutrons and electrons in electric fields On the carbon-12 scale, a proton has a mass of 1.0073, and a neutron a mass of 1.0087.

You need to be confident about this before you go on to the more difficult ideas about the atom which under-pin A'level chemistry.īeyond A'level: Protons and neutrons don't in fact have exactly the same mass - neither of them has a mass of exactly 1 on the carbon-12 scale (the scale on which the relative masses of atoms are measured). This page revises the simple ideas about atomic structure that you will have come across in an introductory chemistry course (for example, GCSE).


 0 kommentar(er)
0 kommentar(er)
